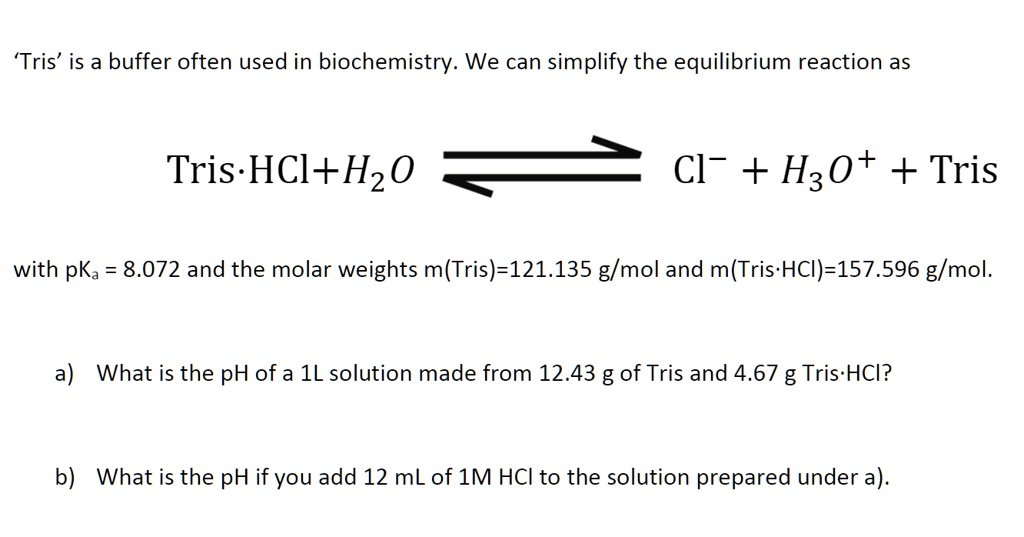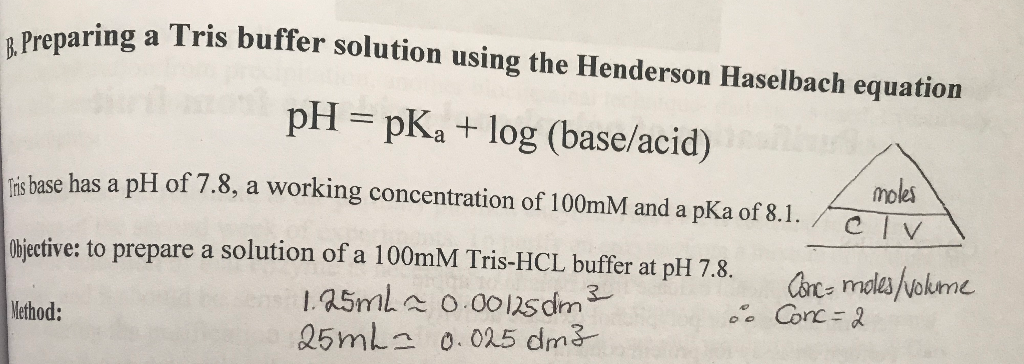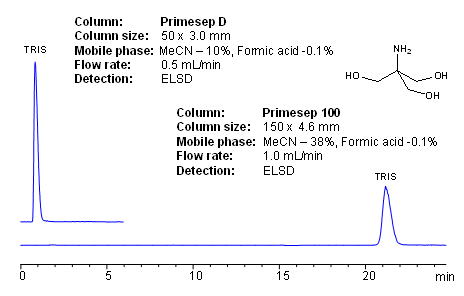
HPLC Method for Analysis of Trometamol (Tris, Tris(hydroxymethyl)aminomethane, Tromethamine, and or THAM) | SIELC Technologies

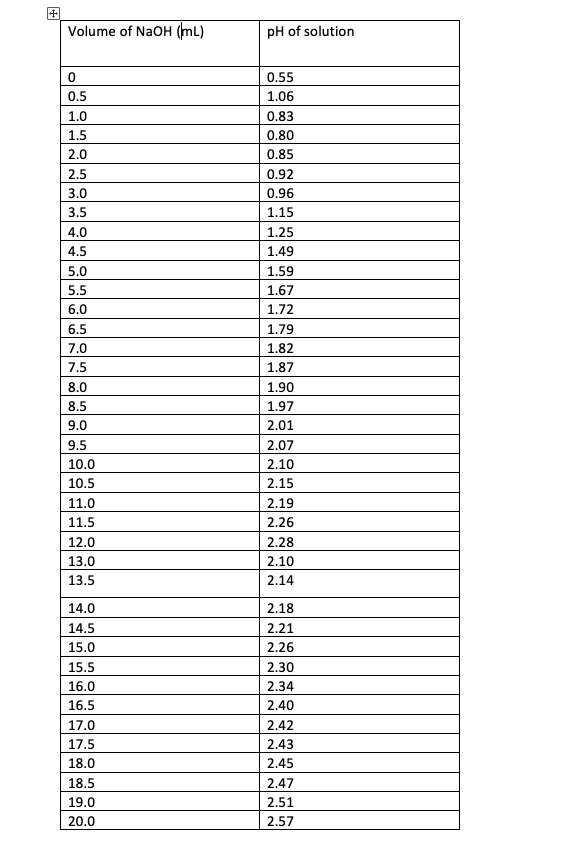
Flood's diagram for three different Tris hydrochloride (pK a ¼ 8.2at 20... | Download Scientific Diagram
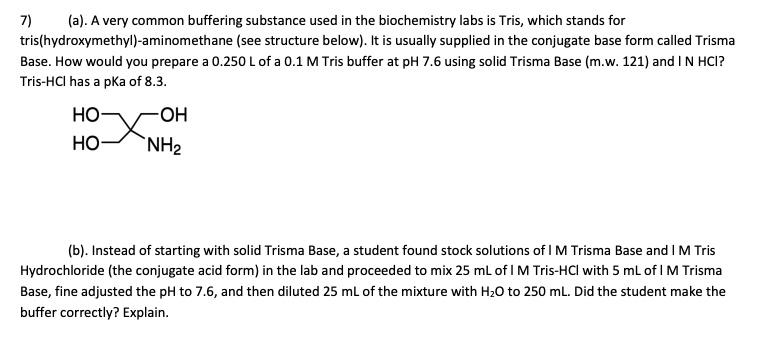
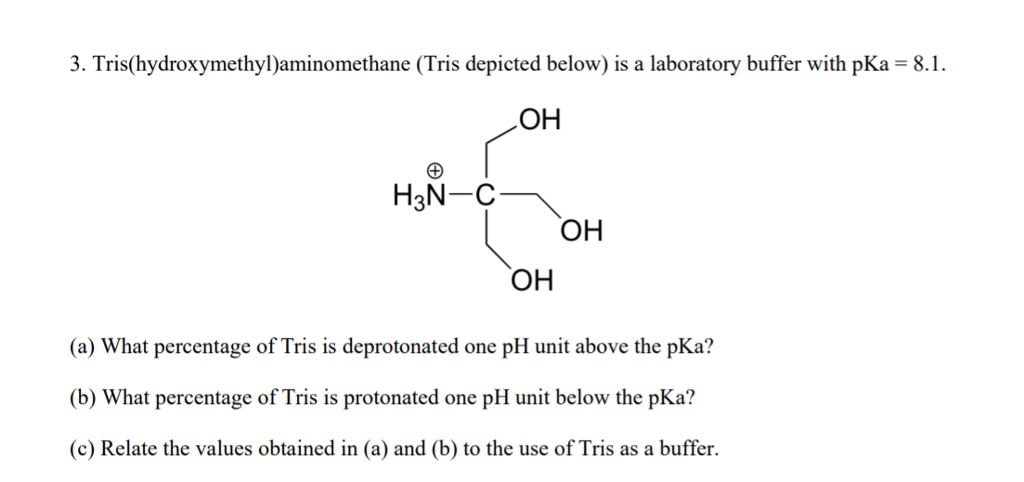
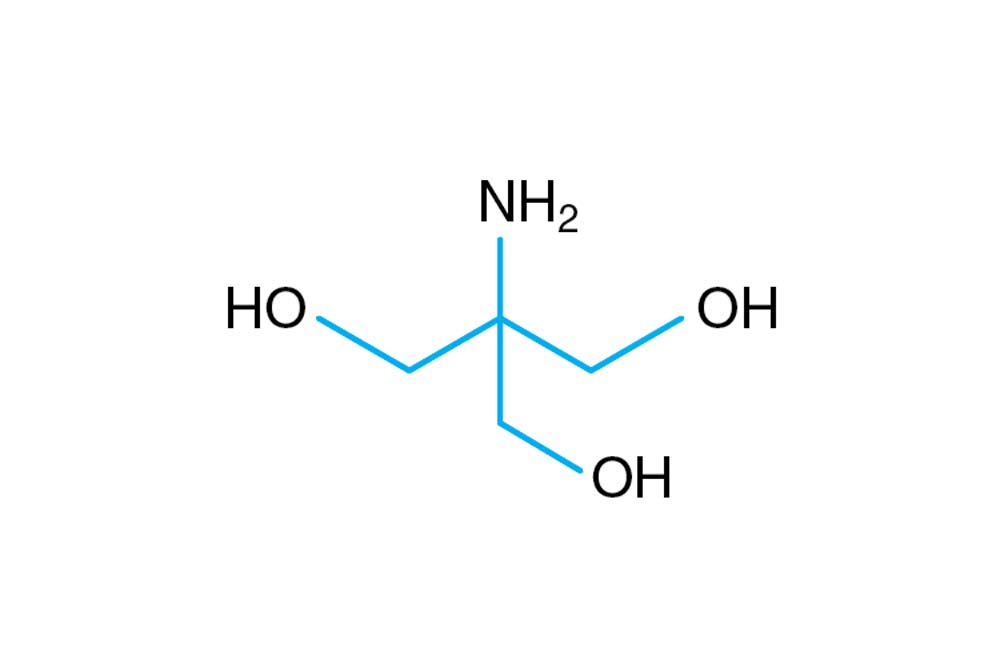
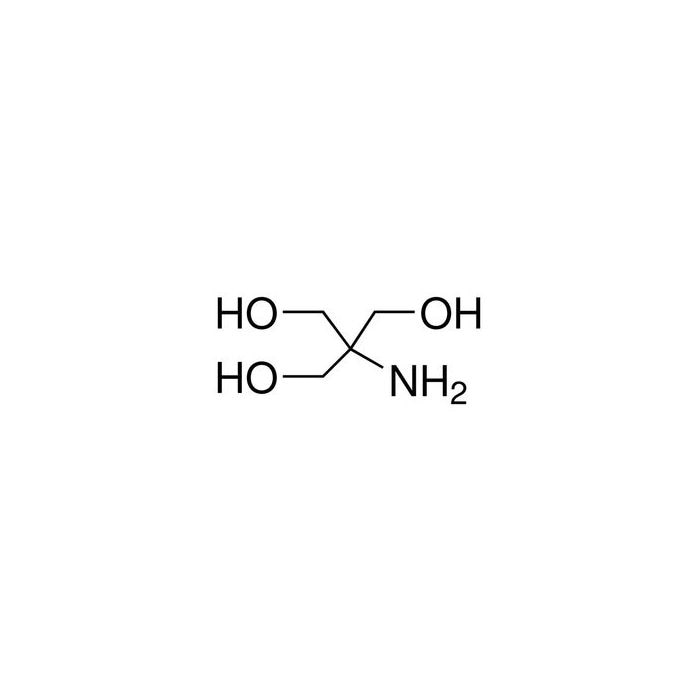
SOLVED: (a) A very common buffering substance used in biochemistry labs is Tris, which stands for tris(hydroxymethyl)aminomethane (see structure below). It is usually supplied in the conjugate base form called Trisma Base .