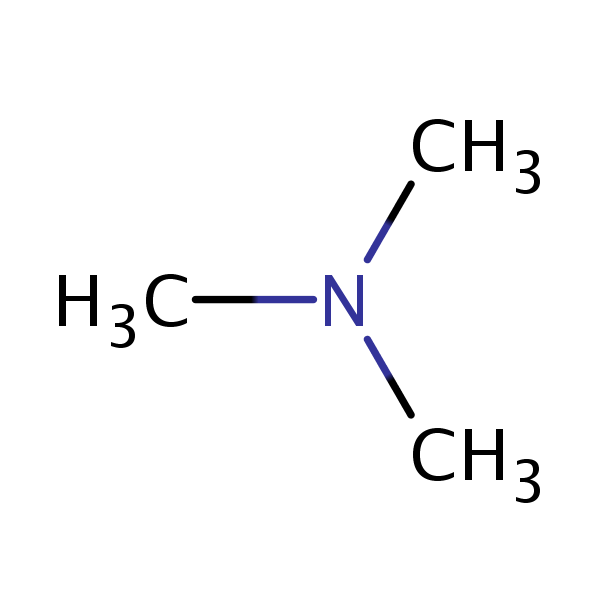
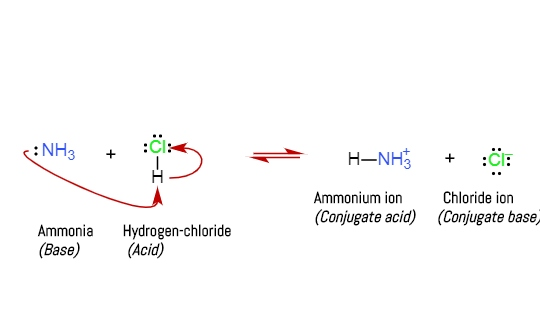
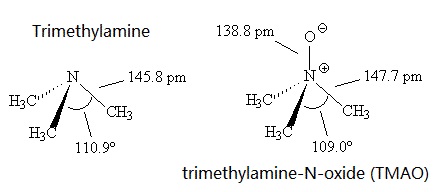
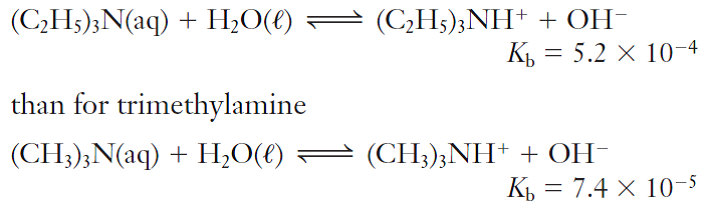Calculate the pH of a 0.443 M aqueous solution of trimethylamine. (Kb = 6.3 x 10-5) | Homework.Study.com
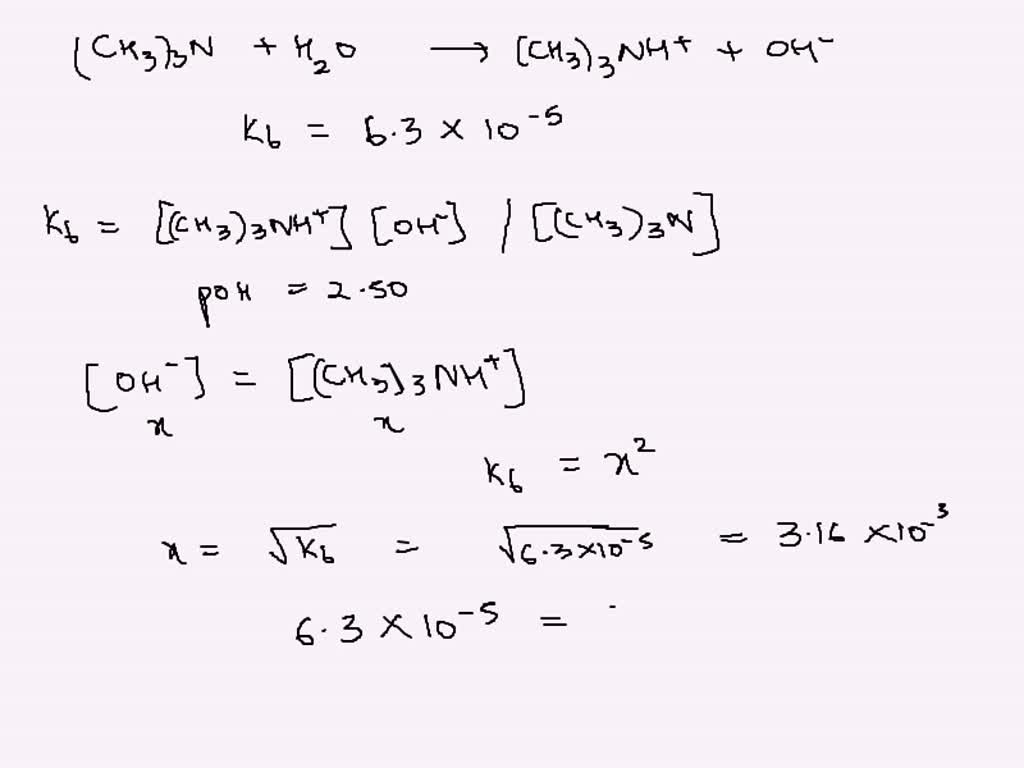
SOLVED: Trimethylamine, (CH3)3N, is a weak base (Kb = 6.3 × 10–5). What volume of this gas, measured at STP, must be dissolved in 2.5 L of solution to give that solution
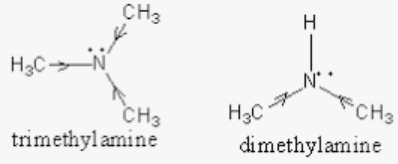
Which among the following is strongest base in gas phase? (a) Triethylamine (b) Diethylamine (c) Ethylamine (d) Ammonia

Trimethylamine Is A Nitrogenous Base And Can Be Protonated To Trimethylammonium Cation. This Colorless, Hygroscopic, And Flammable Tertiary Amine Has A Strong Fishy Or An Ammonia-like Odor Stock Photo, Picture And Royalty