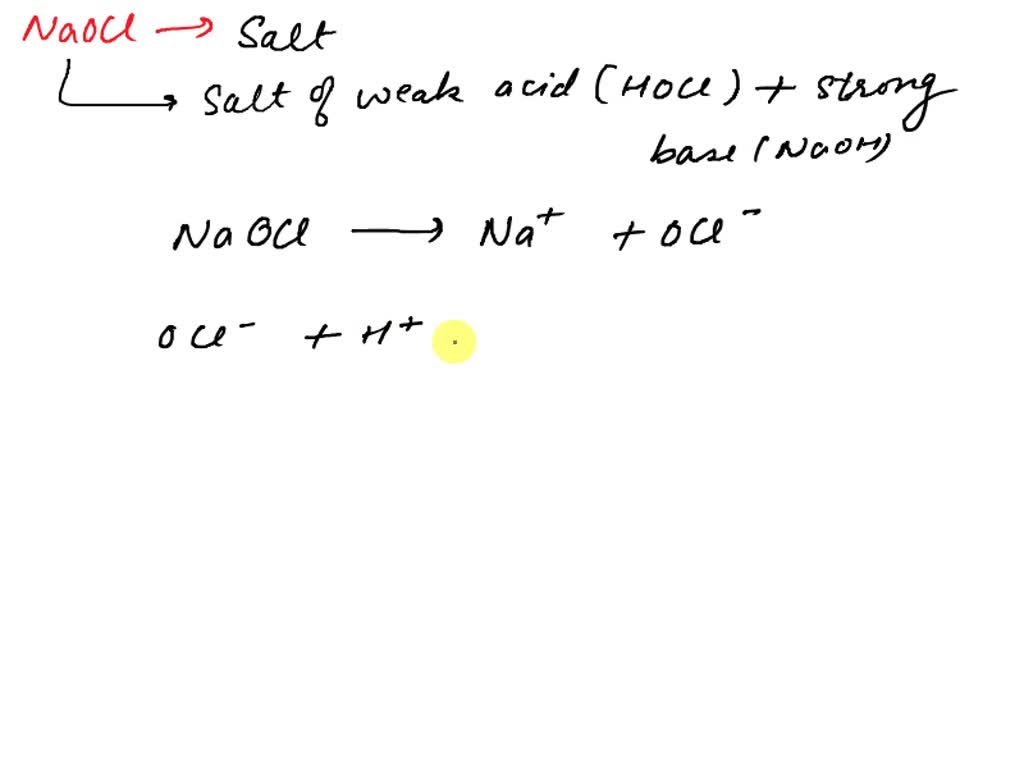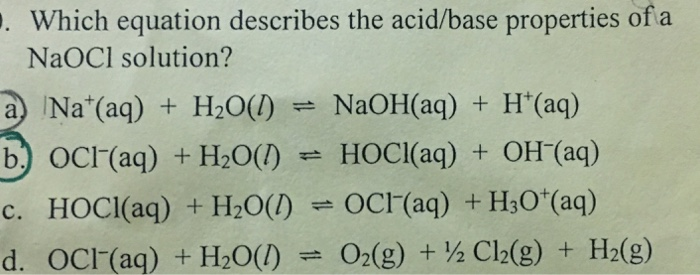Experiment 19: OXIDATION OF 9-FLUORENOL. Objectives: To synthesize a ketone from a secondary alcohol using household bleach. To purify product using. - ppt download

Sodium hypochlorite, NaClO is the active ingredient in many bleaches. Calculate the ratio of the concentration of ClO^- and HClO in a bleach having a pH adjusted to 6.50 by using strong

Why can water act as a base under acidic conditions in organic chemistry mechanisms? - Chemistry Stack Exchange

Oxidation of Alcohols by Sodium Hypochlorite - the Mechanism | Chemistry, Organic chemistry, Alcohol

SOLVED: Match the following salts with the acid-base neutralization reactants that would produce them: NaCIO4 A HCI + Ca(OH)z NaOCl B. HOCI + Ca(OH)z Ca(OCl)z C.HCI + NaOH D.HCIO4 Ca(OH)2 E: HCIO4 +
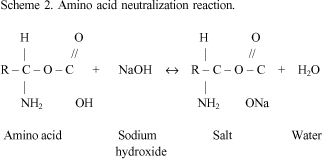
SciELO - Brasil - Mechanism of action of sodium hypochlorite Mechanism of action of sodium hypochlorite