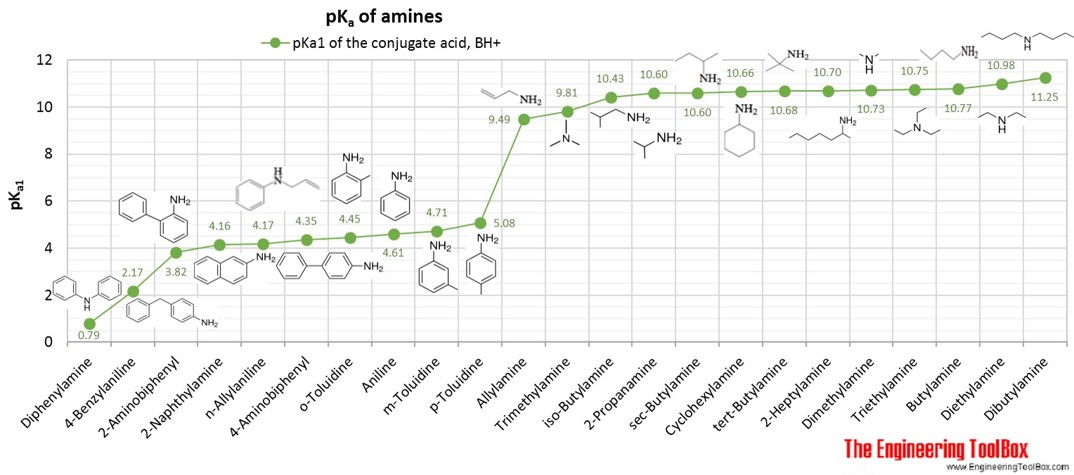
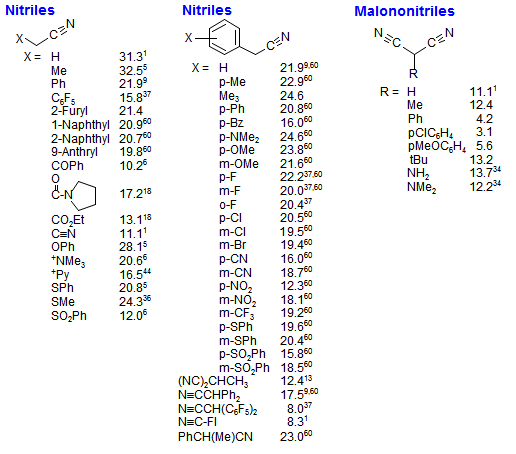
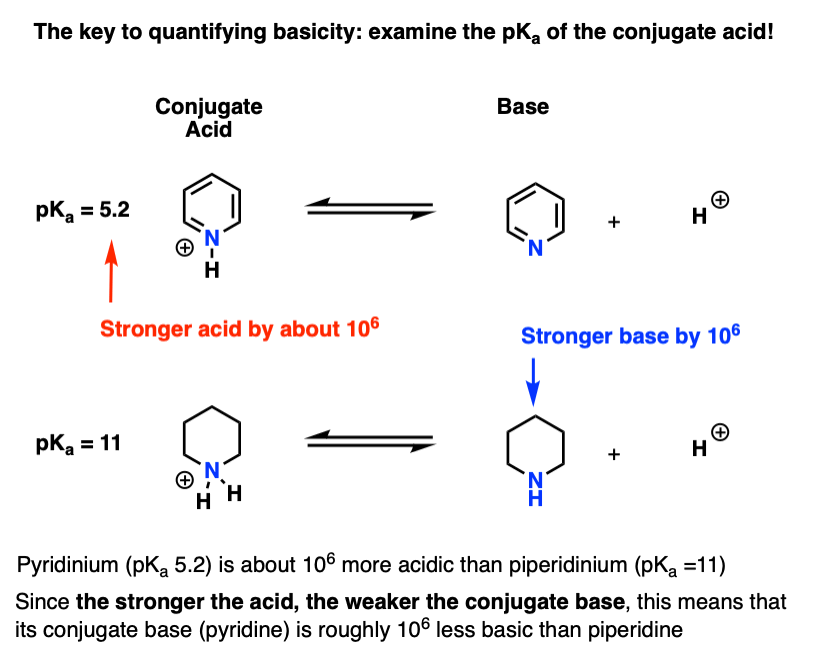
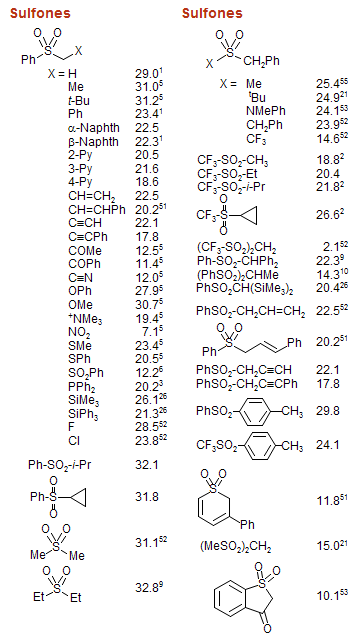Dissociations of free radicals to generate protons, electrophiles or nucleophiles: role in DNA strand breaks
Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis

The Conversion of tert-Butyl Esters to Acid Chlorides Using Thionyl Chloride | The Journal of Organic Chemistry

Novel N-Linked Aminopiperidine Inhibitors of Bacterial Topoisomerase Type II with Reduced pKa: Antibacterial Agents with an Improved Safety Profile | Journal of Medicinal Chemistry

Mono-Oxidation of Bidentate Bis-phosphines in Catalyst Activation: Kinetic and Mechanistic Studies of a Pd/Xantphos-Catalyzed C–H Functionalization | Journal of the American Chemical Society
![PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3c974f939a32262aeed197ab08f1ab631c012982/4-Table4-1.png)