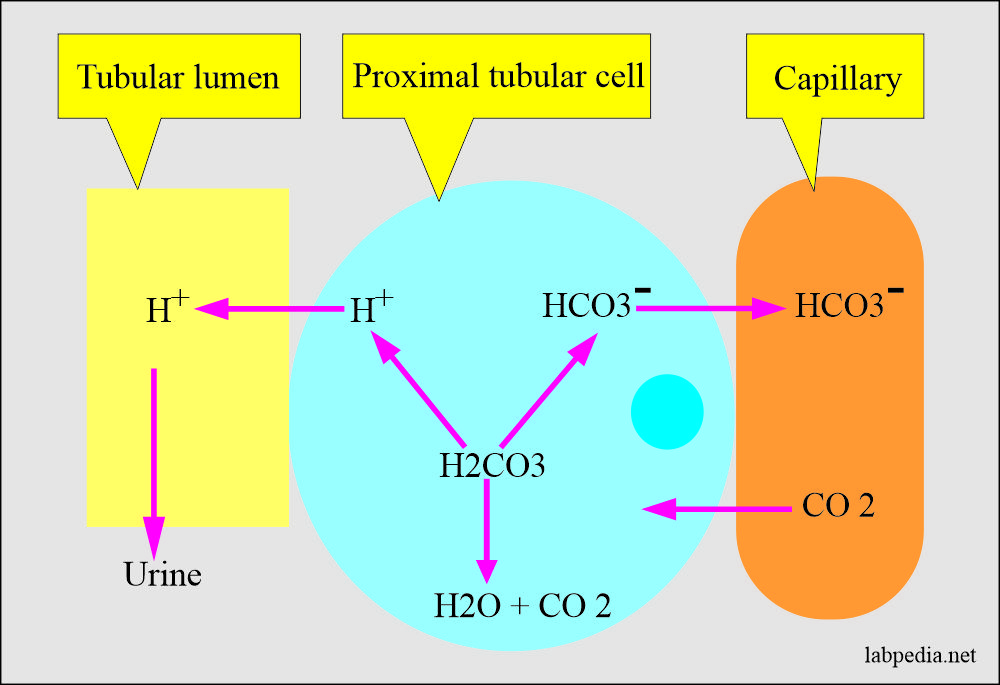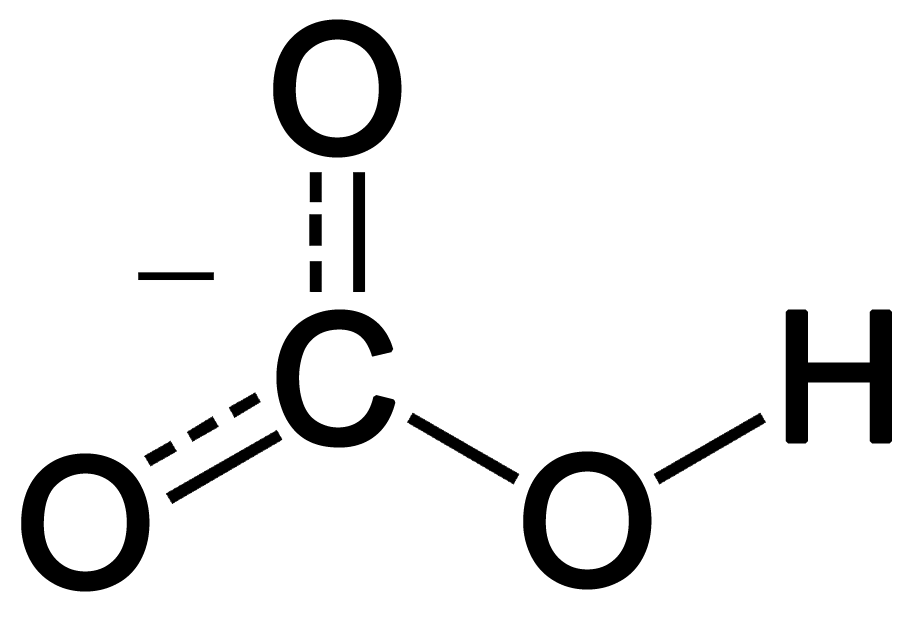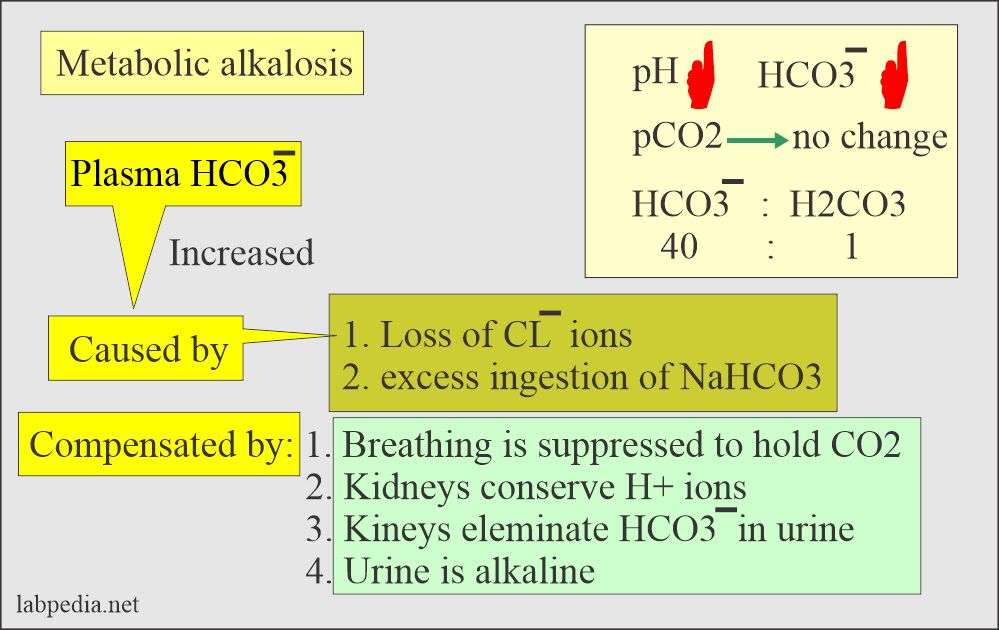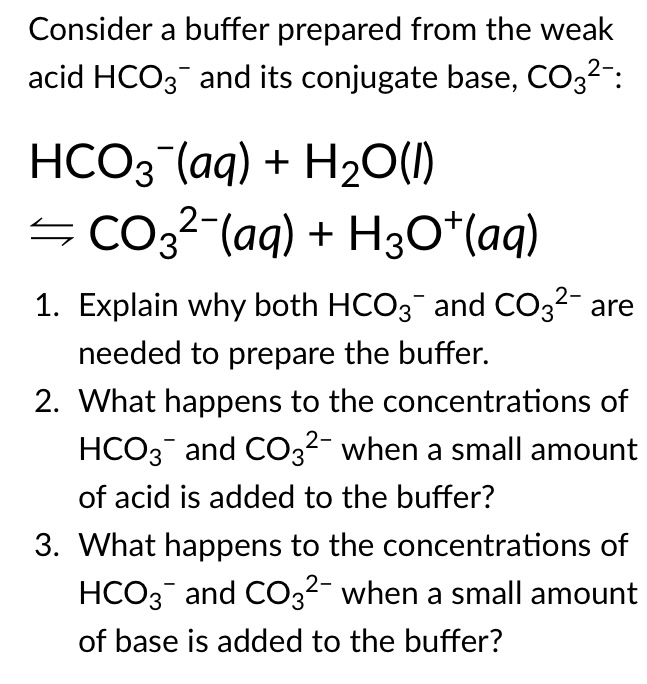
H2CO3(aq)+H2O(l)toHCO3^-(aq) + H3O^+(aq) HCO3^(-)(aq) + H2O(l)to CO3^(2-)(aq)+H3O^(+)(aq) Accoding to the equations above, what is the conjugate base of HCO3^-?

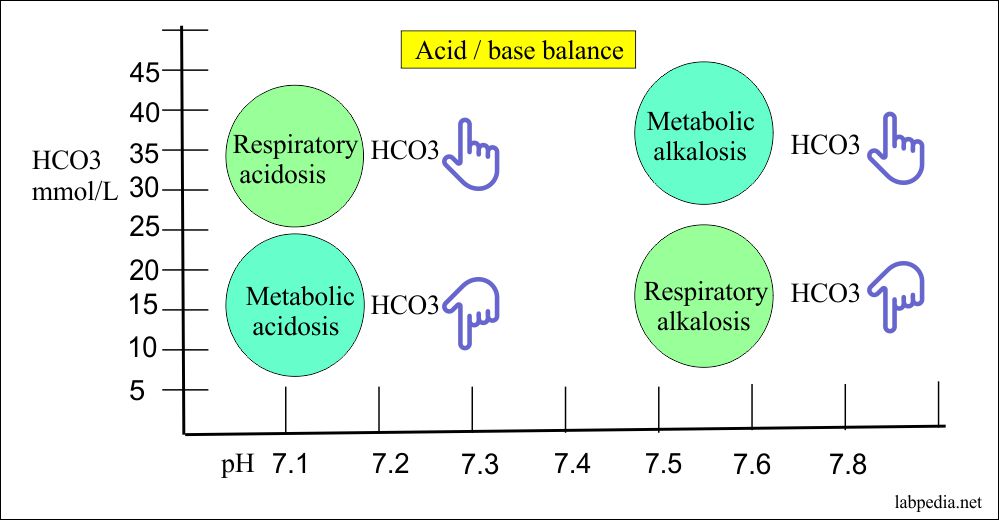
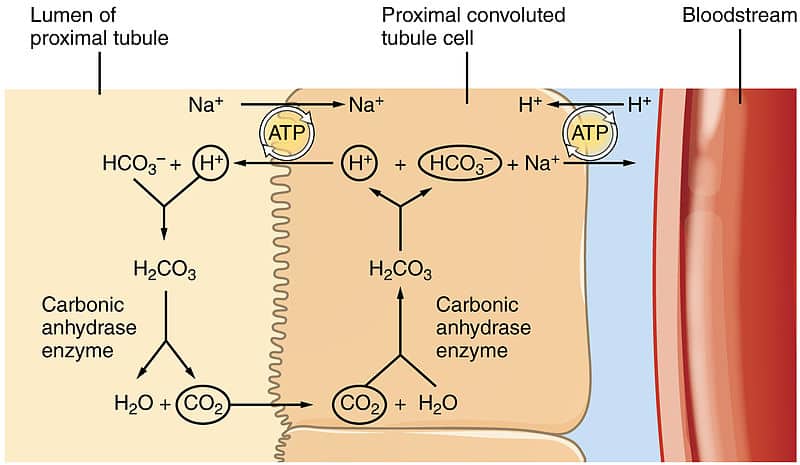
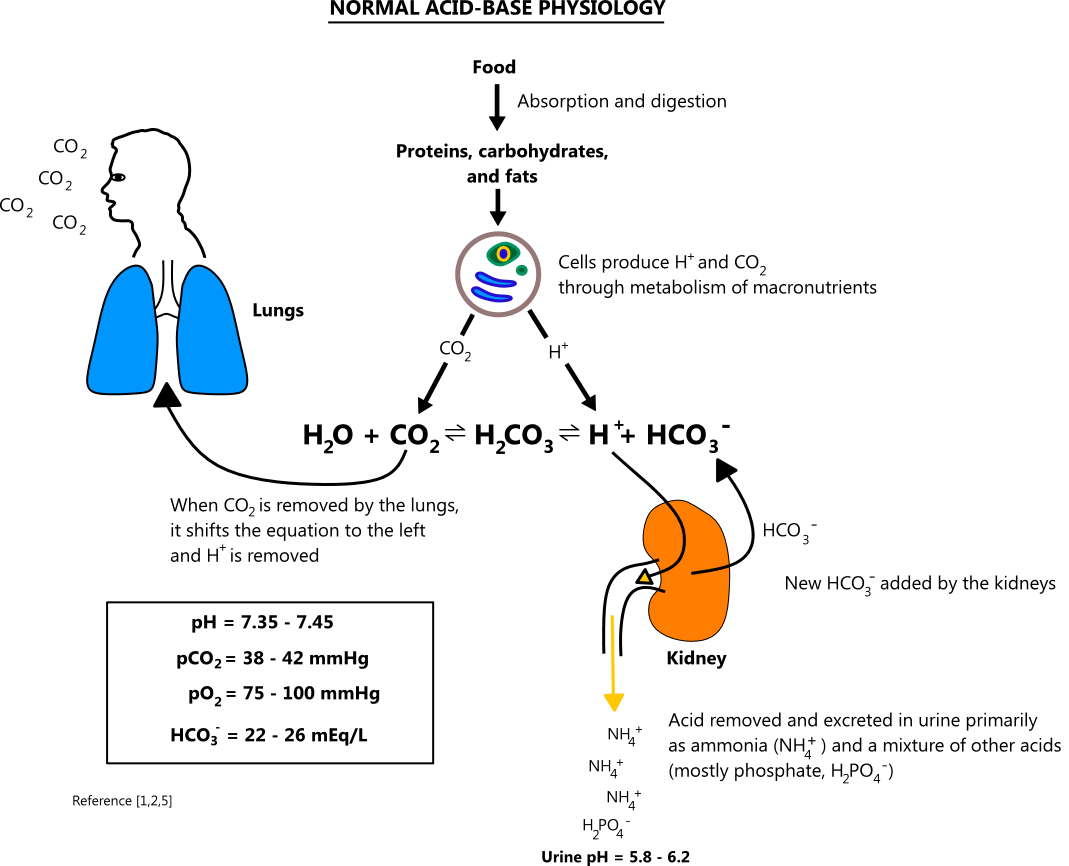
Rishi Kumar, MD - I created this table to teach my trainees how I approach acid-base problems assuming a normal bicarbonate (HCO3) of 24 mmol/L, PaCO2 40 mmHg, arterial pH 7.38-7.42, and

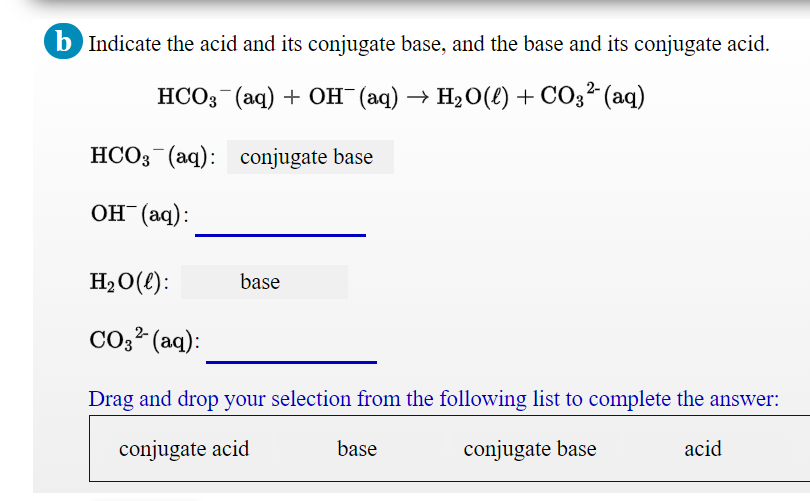
Identify the conjugate acid-base pairs in the following reaction. Indicate what each substance is in each pair. H2CO3 + PO43- arrow HCO3- + HPO42- | Homework.Study.com

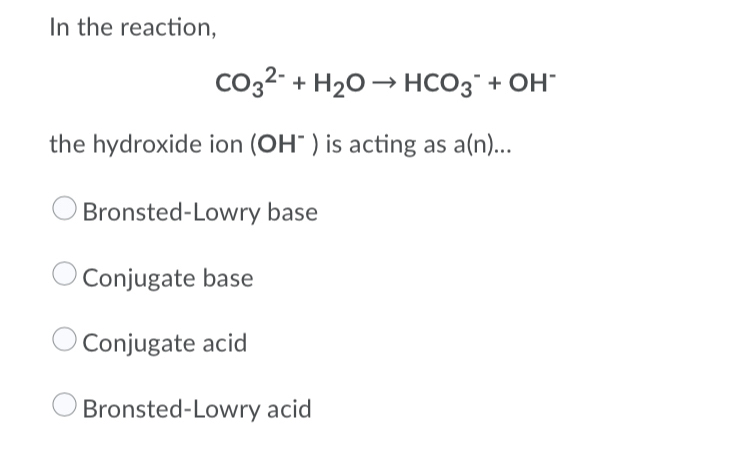
SOLVED: Consider the following reaction. According to Bronsted-Lowry, HCO3- is a(n): HCO3- + H2O —-> H3O+ + CO32- Group of answer choices none of the above base acid conjugate acid conjugate base