Write a chemical equation for the acid-base reaction that occurs when p-phenetidine is dissolved in HCl. Why is HCl used instead of just plain DI water? | Homework.Study.com

pH and Buffers Acids and Bases Acids: H + donors HCl H + + Cl - CH 3 COOH CH 3 COO - + H + Bases: H + acceptors NaOH + H + Na + + H 2 O - ppt download
Set Of Three Chemical Containers With Acid Base And Salt With Different Ph Hcl Hydrochloric Acid Naoh Sodium Hydroxide And Nacl Sodium Chloride Stock Illustration - Download Image Now - iStock

Show how the given species can act as Lewis bases in their reactions with HCl. CH_3CH_2OH, (CH_3)_2NH, (CH_3)_3P | Homework.Study.com

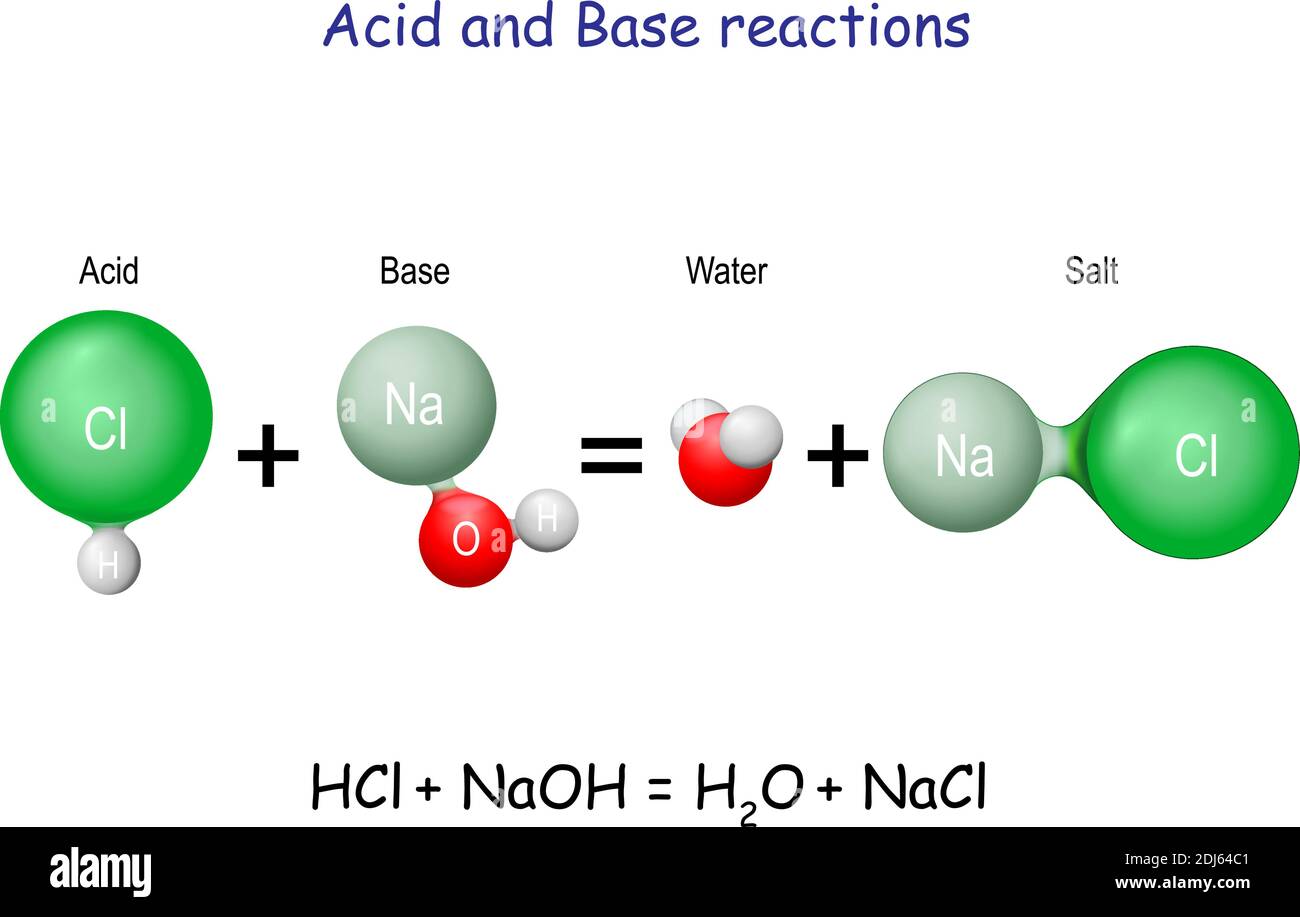
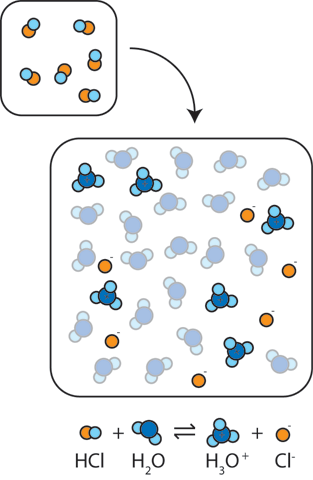
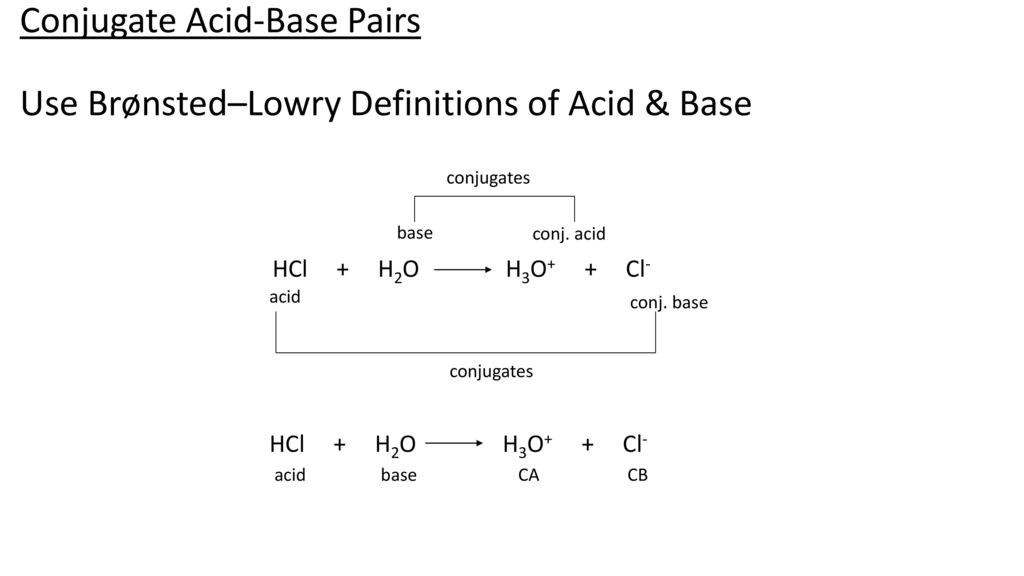
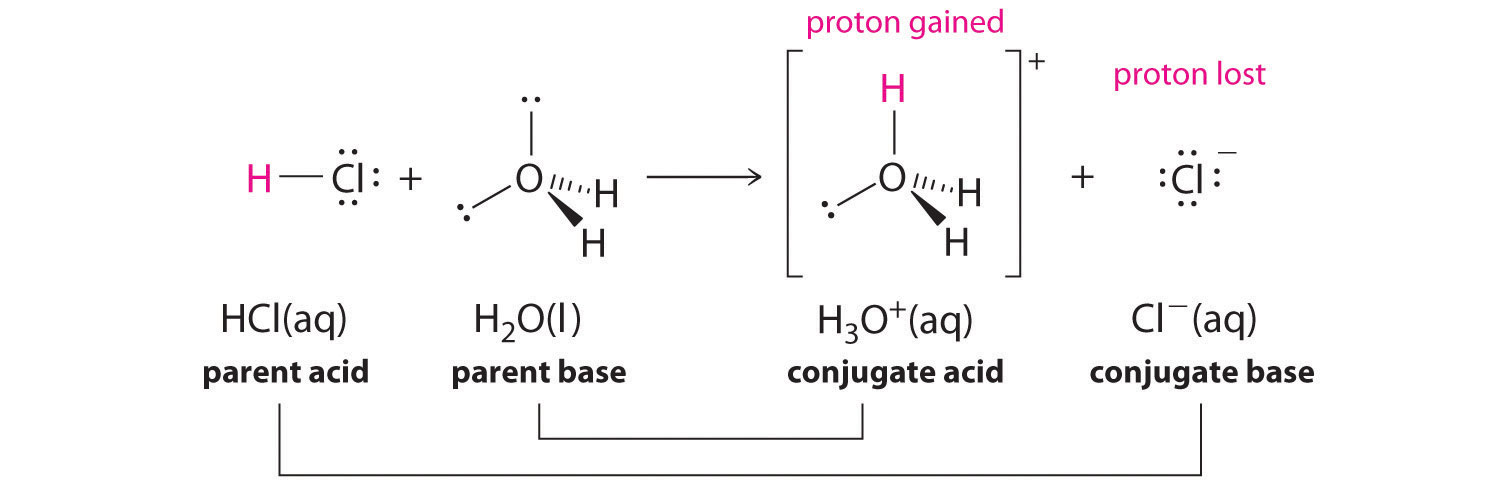
The ionization of hydrochloric acid in water is given below: HCl(aq) + H2O(l) H3O^+(aq) + Cl^-(aq) Lable two conjugate acid - base pairs in this ionization.