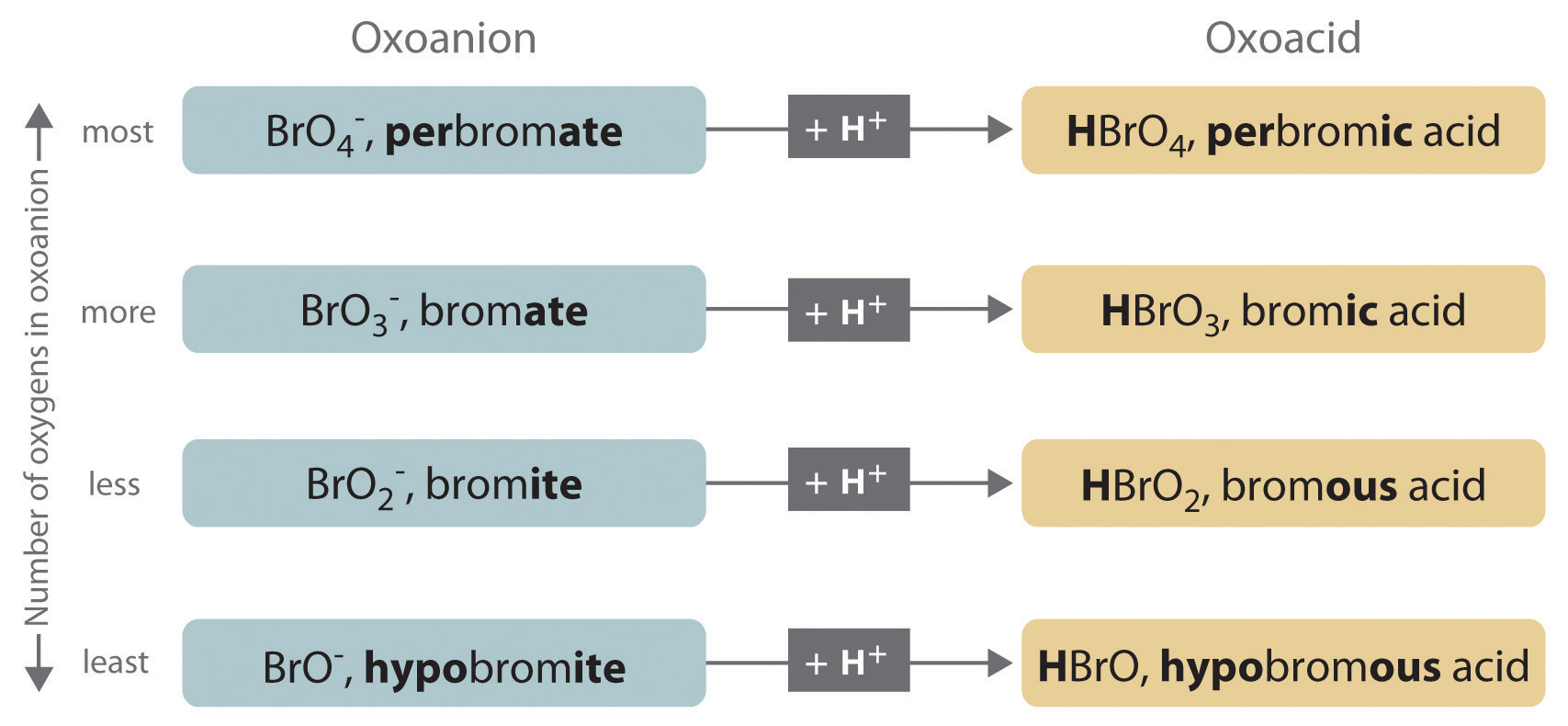
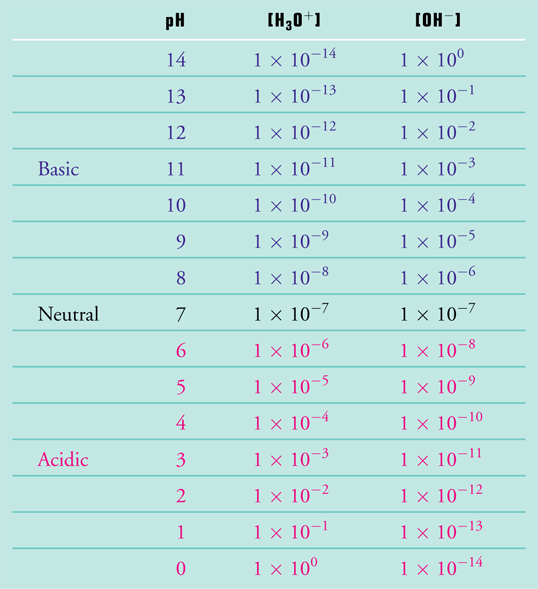
Lesson 5: Determining the strength of an acid or base - aeterniti | Chemistry | Vingle, Interest Network

Determining PH of a Solution | Acidic, Basic & Neutral Solutions - Video & Lesson Transcript | Study.com

Identifying Acids and Bases Acids Acid (anhydrides) Bases Base (anhydrides) Salts contains H+ ions as the cation, with and other element as the anion. - ppt download




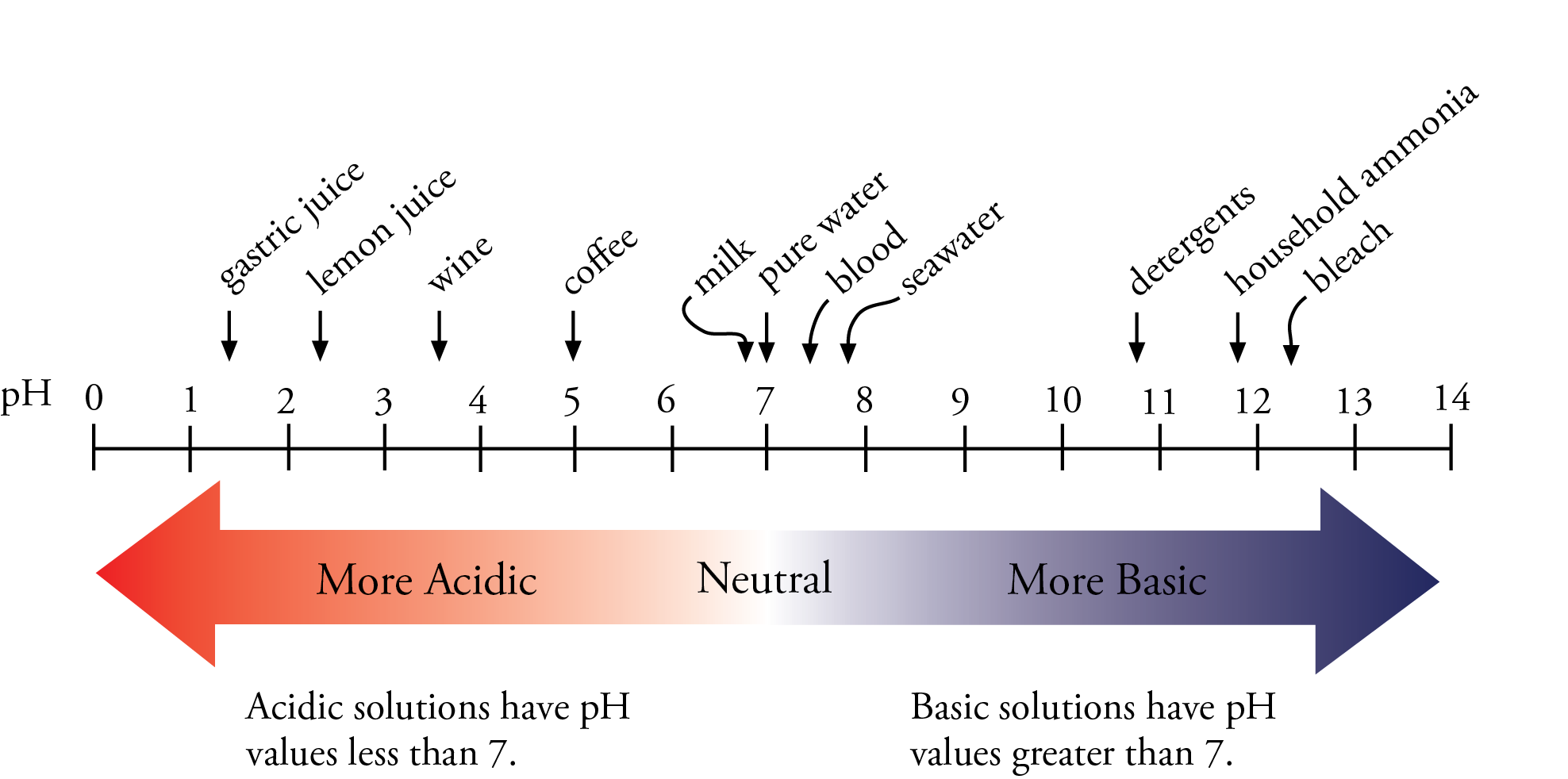
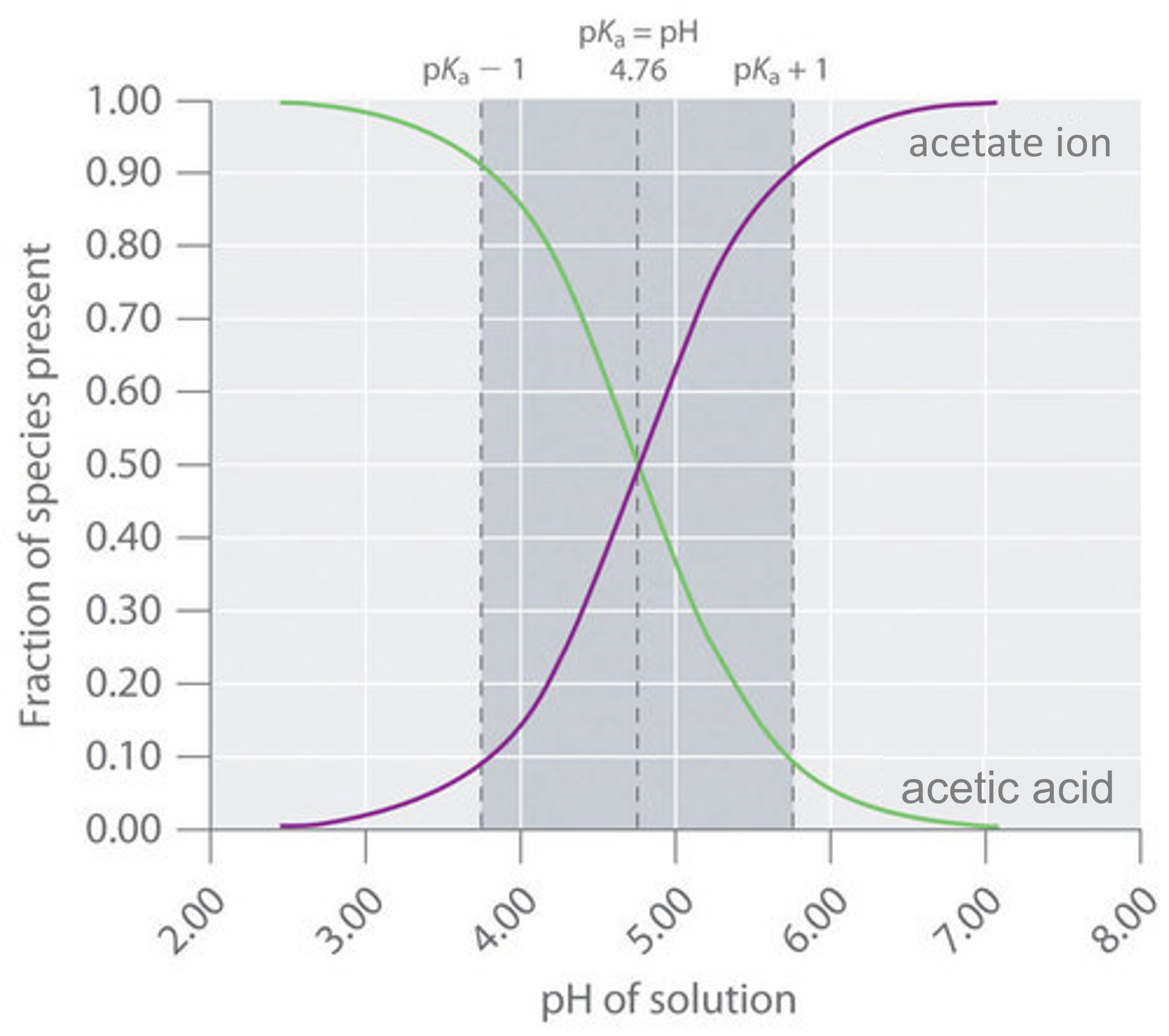
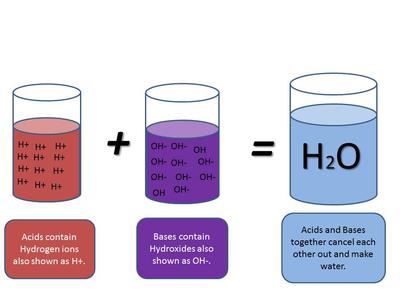


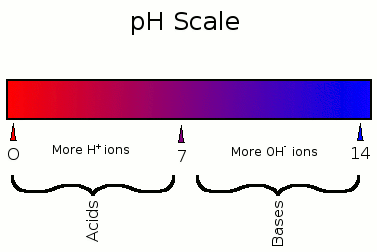
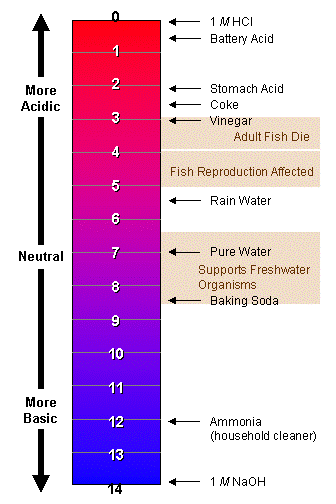
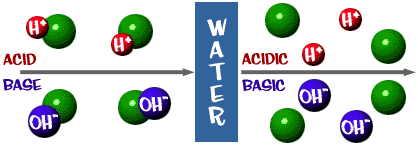
![Calculating pH, pOH, [H+], [OH-] - Acids and Bases Calculating pH, pOH, [H+], [OH-] - Acids and Bases](http://iloveacid--basechemistry.weebly.com/uploads/2/7/8/0/27808151/2222607_orig.gif)