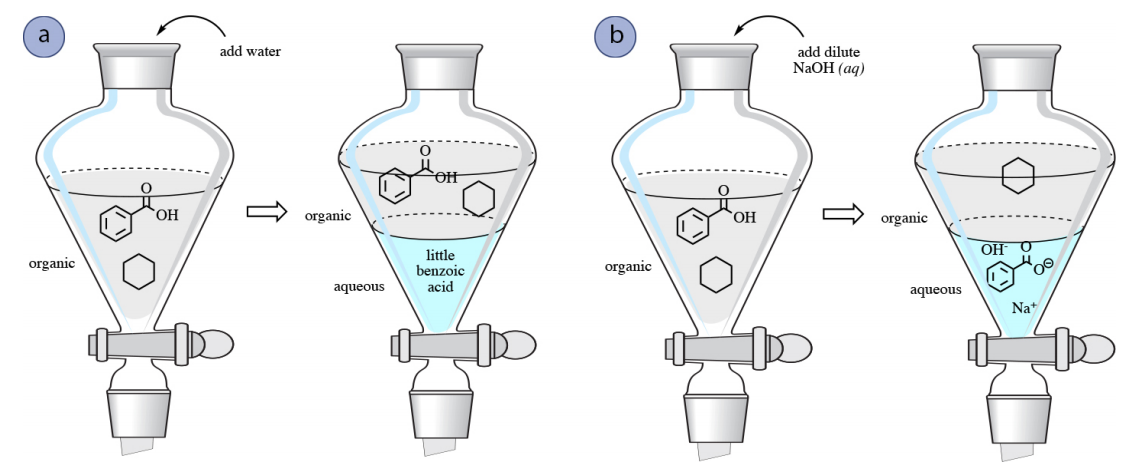
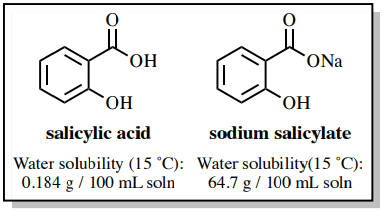
OneClass: Benzoic acid is soluble in diethyl ether but not water, however, benzoic acid is extracted ...

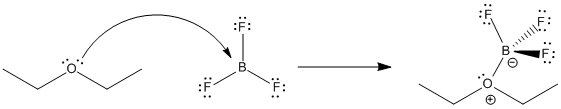
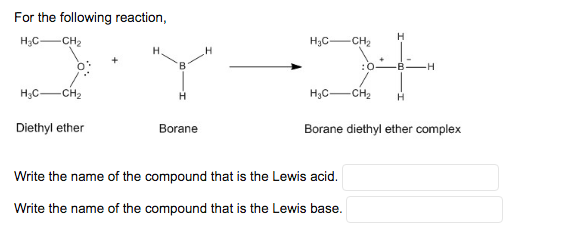
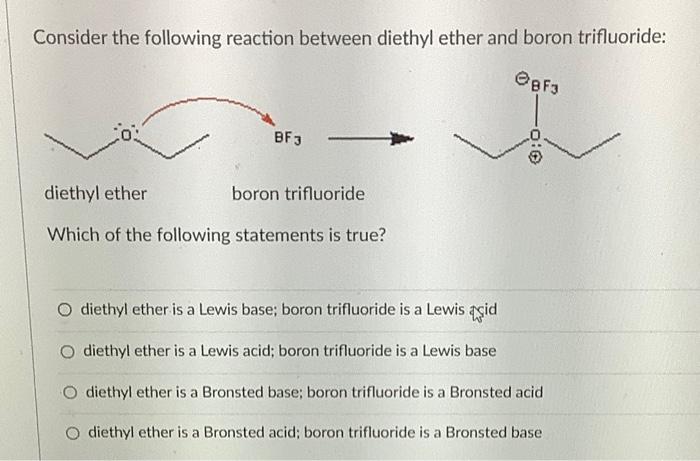
CH_3CH_2OCH_2CH_3 is best classified as a ______. A) Bronsted-Lowry acid. B) Lewis acid. C) Bronsted-Lowry base. D) Lewis base. E) Both C & D. | Homework.Study.com

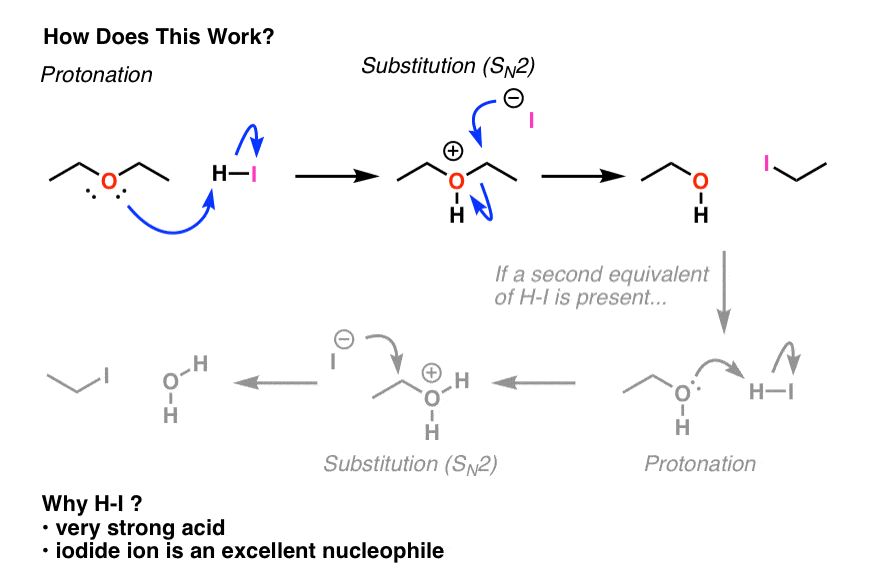
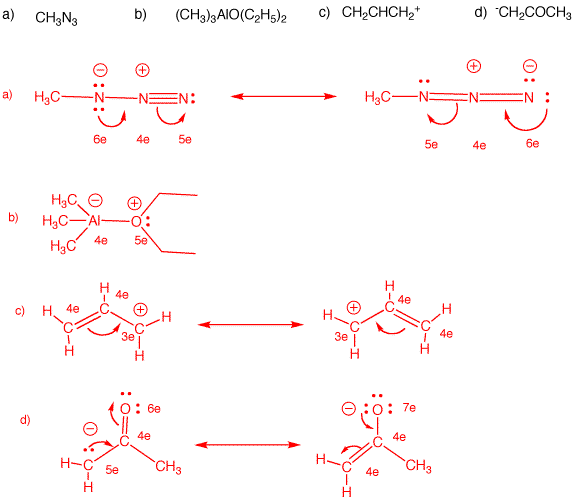
Why each of the three solids(acid, base and neutral)soluble in diethyl ether? examine the structure of of each solid and explain what factors increase solubility in water vs the organic solvent used.

SOLVED: Phenol is soluble in diethyl ether but not water; however , phenol is extracted from diethyl ether with aqueous sodium hydroxide Complete the acid-base reaction below by writing the products of