Structure of the PILs' precursors: DBU base (a) and the three acids... | Download Scientific Diagram
Proposed mechanisms for the DBU catalyzed urea-synthesis reaction from... | Download Scientific Diagram

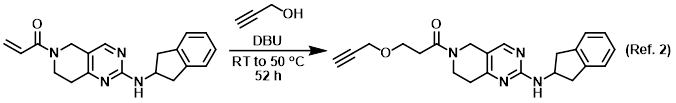
DBU‐Promoted Nucleophilic Activation of Carbonic Acid Diesters - Carafa - 2011 - European Journal of Organic Chemistry - Wiley Online Library
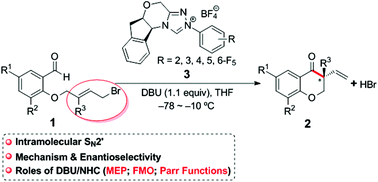
Theoretical study on the mechanism and enantioselectivity of NHC-catalyzed intramolecular SN2′ nucleophilic substitution: what are the roles of NHC and DBU? - Organic Chemistry Frontiers (RSC Publishing)
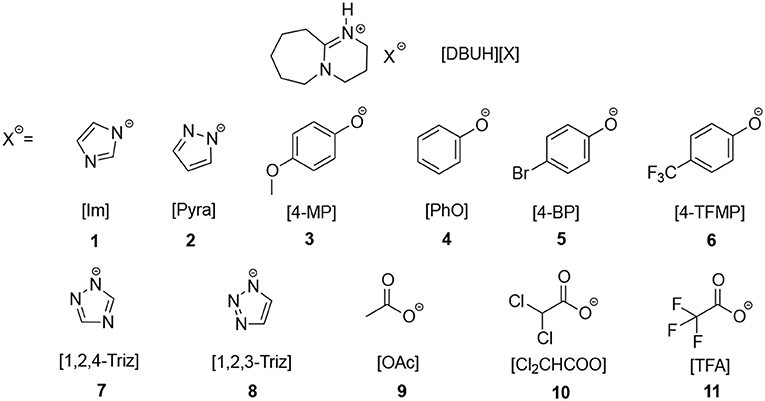
Frontiers | CO2 Absorption by DBU-Based Protic Ionic Liquids: Basicity of Anion Dictates the Absorption Capacity and Mechanism
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters](https://pubs.acs.org/cms/10.1021/acs.orglett.5b02398/asset/images/large/ol-2015-02398j_0002.jpeg)
The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters](https://pubs.acs.org/cms/10.1021/acs.orglett.5b02398/asset/images/medium/ol-2015-02398j_0006.gif)
The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters

DBU‐Promoted Intramolecular Crossed Aldol Reaction: A Facile Access to Indane‐Fused Pyrrolidine - Yang - 2019 - European Journal of Organic Chemistry - Wiley Online Library
![Mechanistic investigation-inspired activation mode of DBU and the function of the α-diazo group in the reaction of the α-amino ketone compound and EDA: [DBU-H]+-DMF-H2O and α-diazo as strong N-terminal nucleophiles - ScienceDirect Mechanistic investigation-inspired activation mode of DBU and the function of the α-diazo group in the reaction of the α-amino ketone compound and EDA: [DBU-H]+-DMF-H2O and α-diazo as strong N-terminal nucleophiles - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S2052411022024750-c9qo00602h-ga.jpg)
Mechanistic investigation-inspired activation mode of DBU and the function of the α-diazo group in the reaction of the α-amino ketone compound and EDA: [DBU-H]+-DMF-H2O and α-diazo as strong N-terminal nucleophiles - ScienceDirect
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters](https://pubs.acs.org/cms/10.1021/acs.orglett.5b02398/asset/images/large/ol-2015-02398j_0001.jpeg)
The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters
Synthesis of five- and six-membered heterocycles by dimethyl carbonate with catalytic amounts of nitrogen bicyclic bases - Green Chemistry (RSC Publishing) DOI:10.1039/C4GC01822B
![Facile synthesis of 2-arylimidazo[1,2-a]pyridines catalysed by DBU in aqueous ethanol | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences Facile synthesis of 2-arylimidazo[1,2-a]pyridines catalysed by DBU in aqueous ethanol | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences](https://royalsocietypublishing.org/cms/asset/51bddc39-45b7-4fc6-865b-a440147711d1/rspa20190238f03.gif)
Facile synthesis of 2-arylimidazo[1,2-a]pyridines catalysed by DBU in aqueous ethanol | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences

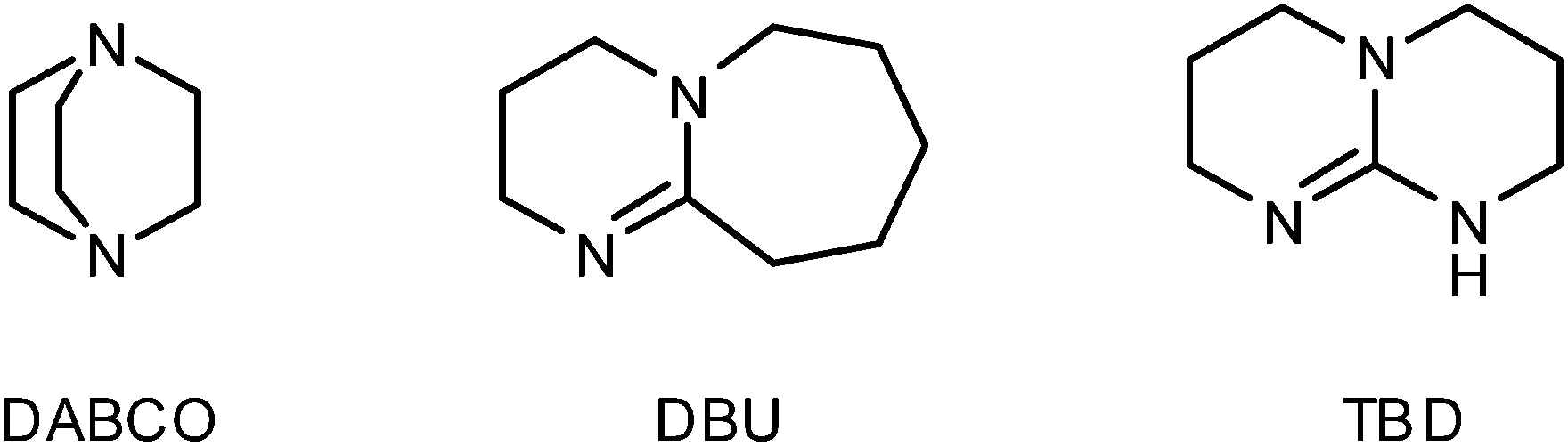
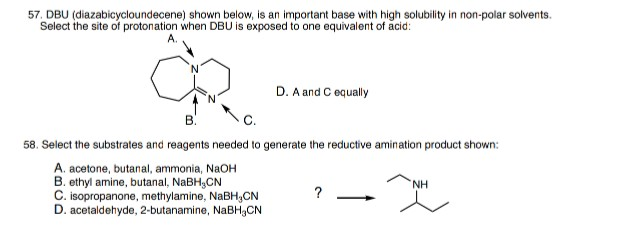
DBU, 1,8-diazabicyclo 5.4.0 undec-7-ene, is a base in elimination reactions. Which N atom is more basic in DBU? Explain your choice. | Homework.Study.com
![Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/da071cf8-eee0-4e2f-8e22-5b6f6a0f91e7/adsc201601279-fig-5004-m.jpg)

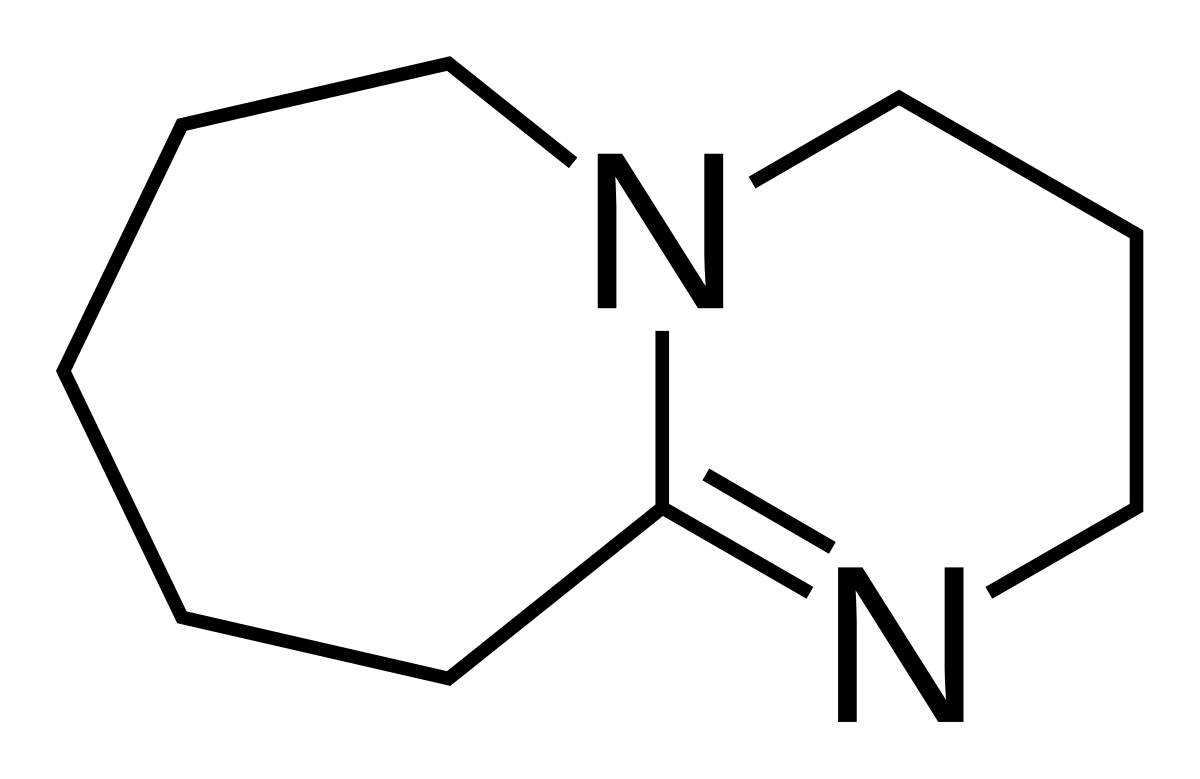




![1,8-Diazabicyclo[5.4.0]undec-7-ene | C9H16N2 - PubChem 1,8-Diazabicyclo[5.4.0]undec-7-ene | C9H16N2 - PubChem](https://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=81184&t=l)

![DBU [1,8-Diazabicyclo[5,4,0]undec-7-ene] - An Overview - YouTube DBU [1,8-Diazabicyclo[5,4,0]undec-7-ene] - An Overview - YouTube](https://i.ytimg.com/vi/fu67cE_ydew/maxresdefault.jpg)