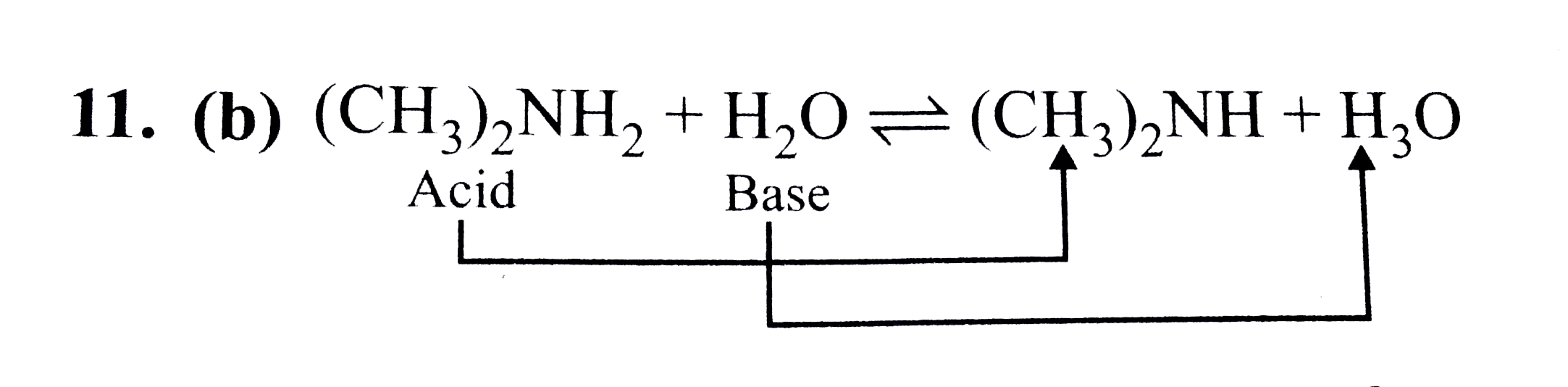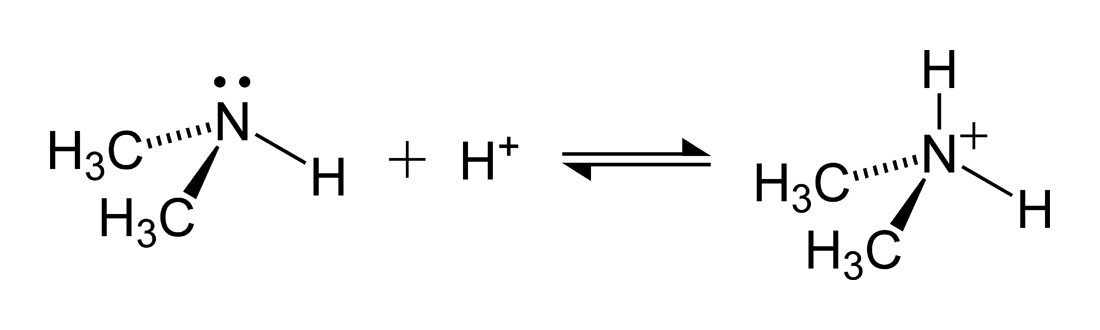SOLVED: 1.) A 21.4 mL sample of 0.271 M dimethylamine, (CH3)2NH, is titrated with 0.372 M nitric acid. After adding 23.5 mL of nitric acid, the pH is . 2.) A 29.4

Draw the product formed when the Lewis acid (CH3CH2)3C reacts with the Lewis base (CH3)2NH. - Brainly.com
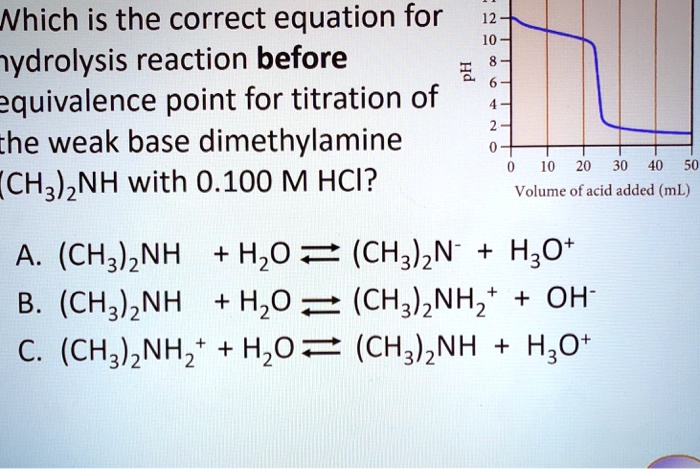
SOLVED: Nhich is the correct equation for ydrolysis reaction before 2 quivalence point for titration of he weak base dimethylamine (CHz)NH with 0.100 M HCI? Volume of acid added (mL) A (CH3)zNH

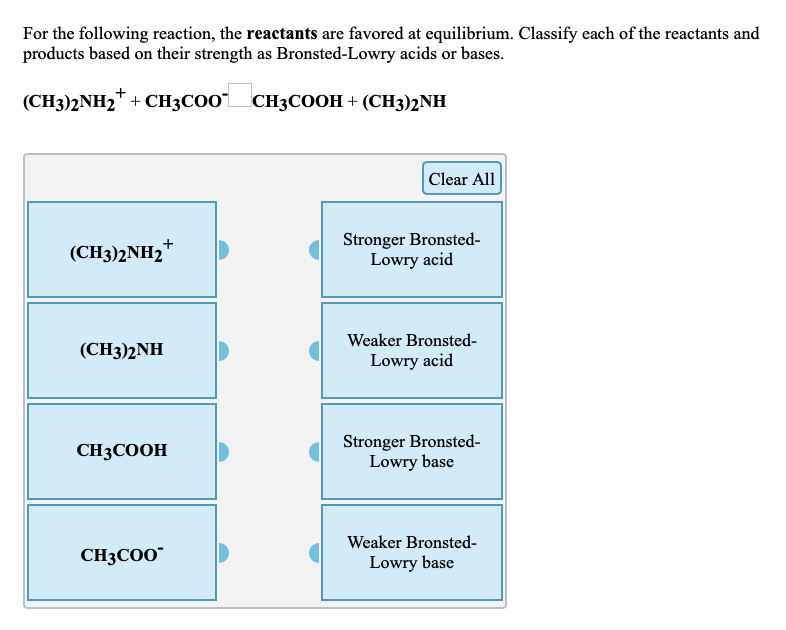
SOLVED: For the following reaction, K < 1. Classify each of the reactants and products based on their strength as Bronsted-Lowry acids or bases. (CH3)2NH2+ + CN- –> (CH3)2NH + HCN CN-
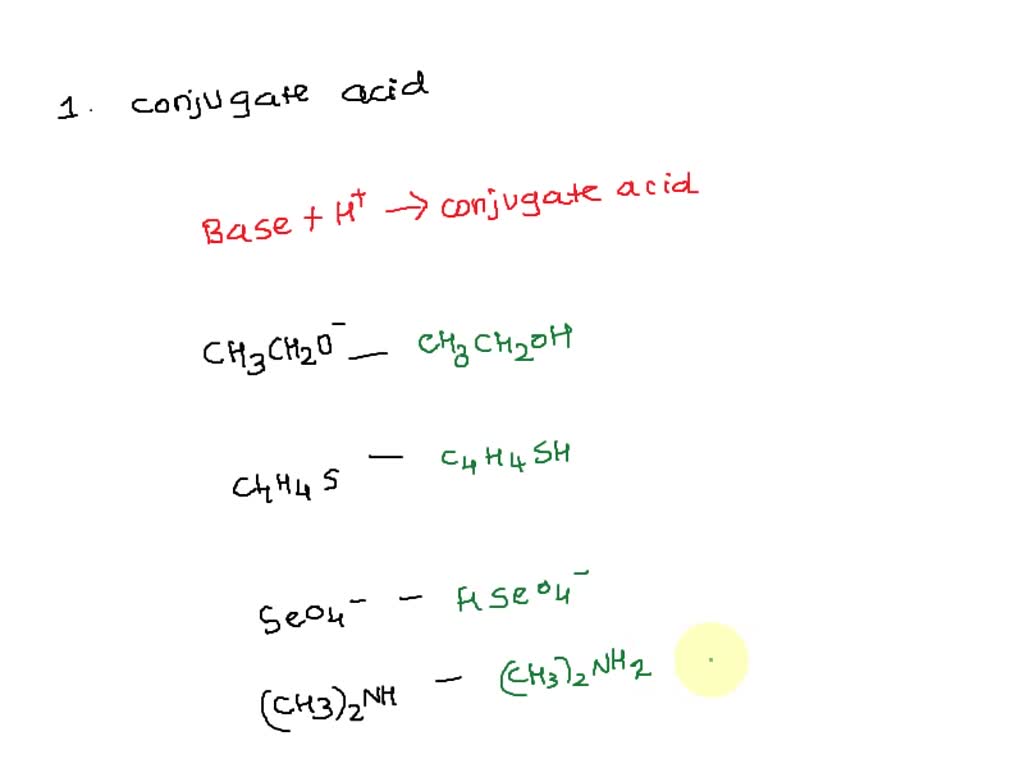
SOLVED: Q: 1). Write the formulas of the conjugate acids of the following Brønsted-Lowry bases. - CH3CH2O− - C4H4S - SeO-24 - (CH3)2NH 2). Enter the formulas of the conjugate bases of

What is the order of basicity of the following compounds? CH3NH2, (CH3)2NH, (CH3)3N (in protic solvent)

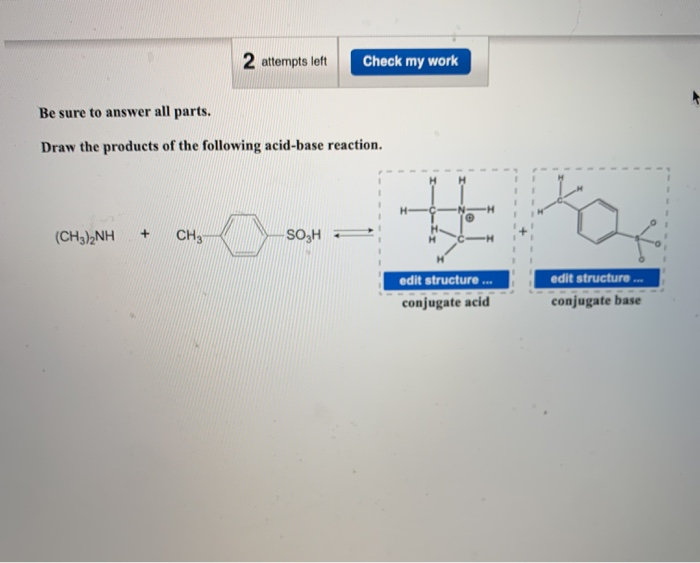
Provide the structure of the conjugate acid and the conjugate base of dimethylamine ((CH3)2NH). | Homework.Study.com